Electrifying membrane synthesis
In 2018, we reported the first example of electrified MOF membrane synthesis, where a conductive porous support was used directly as the cathode and a continuous zeolitic imidazolate framework-8 (ZIF-8) membrane could be obtained within only 20 min. The role of the external current during the electrified growth of MOF membranes is to promote the ligand deprotonation process. Notably, ligand deprotonation is of prime importance in MOF chemistry and is the prerequisite of all the ensuing coordination reactions. Traditional approaches for this step rely on the in situ base generated from the solvent dimethylformamide (DMF) during the solvothermal growth and the subsequent acid–base reaction, which requires may require a long reaction period of several hours to several days. However, when this step is replaced by the electrochemically assisted process, the rate is dramatically accelerated. During the electrified synthesis of ZIF-8 membranes as well as the associated derivative multivariate MOF membranes, the ligands are first deprotonated driven or assisted by the electrons from the support surface, and then the resulting deprotonated ligands are accumula are expected to migrate toward attracted to the support surface under the driving force from the electric field. The metal nodes with the desired connectivity could coordinate with the deprotonated ligands to form MOF membranes on the support surface.
Related papers
- Nature 606 (7915), 706-712
- Nature Energy 6 (9), 882-891
- Science advances 4 (10), eaau1393
Separation of hydrocarbon mixtures
Olefins purification from the olefin/paraffin mixtures is one of the most energy-intensive processes in industry and innovative separations are needed. Separation of short-chain olefin/paraffin mixtures is currently dominated by cryogenic distillation, which consumes over 120 TBtu per year. Commonly, a two-column based system with more than 200 trays is required to extract high-purity propene (at least 99.5 weight % for polymer-grade specifications) for PP production. The columns usually range from 70~90 m in height and 2~6 m in diameter, working with a high reflux ratio of about 15~25 and a tray efficiency of 70%~80%, which makes this process extremely energy expensive. Successful implementation of membranes for the challenging hydrocarbons separation will contribute to at least 25% capital saving pertaining to the propylene/propane separation. We designed different types of MOF membranes to address the energy-efficient separation of hydrocarbons, which could help save up to 89% of energy consumptions compared with the traditional distillation methods.
Related papers
- Nature Energy 6 (9), 882-891
- Science advances 4 (10), eaau1393
- J. Am. Chem. Soc. 142 (21), 9582-9586
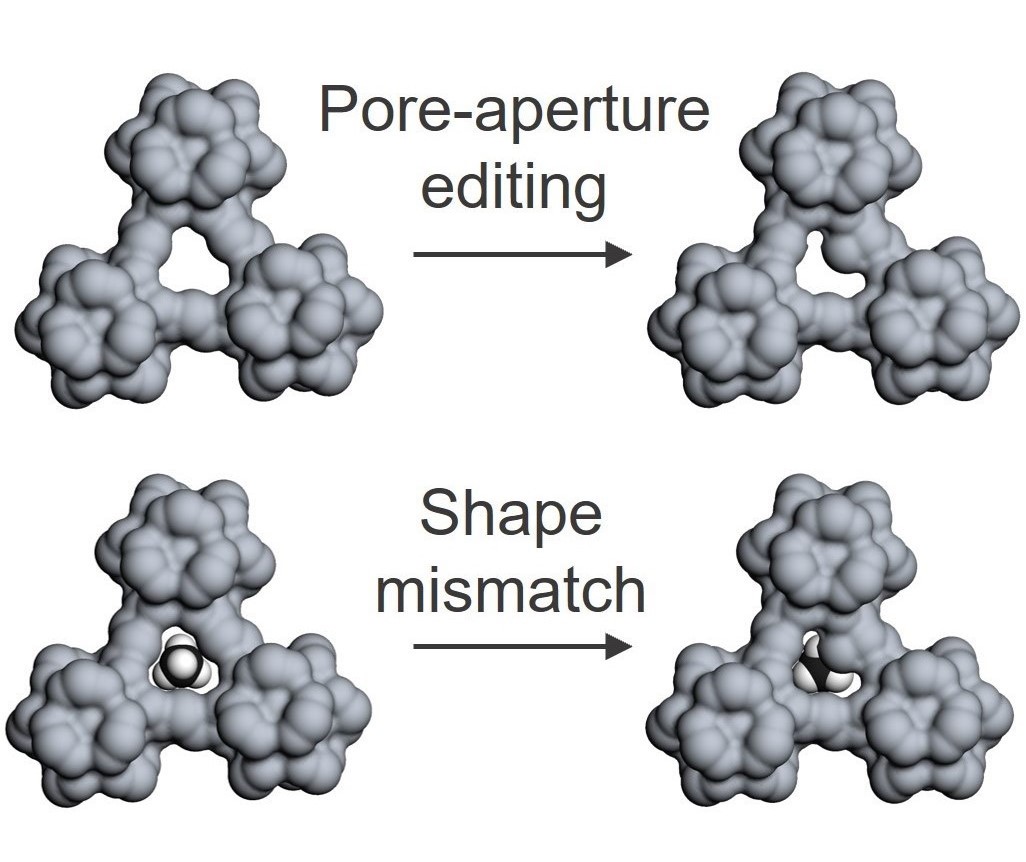
Shape-mismatch induced separation
When researchers aim to fabricate a gas-separation membrane, they usually check the kinetic size of each gas component and then look for a material with a suitable pore size. This routine logic makes sense in most scenarios, but not always — as shown by the problem of nitrogen–methane separation. We have designed a porous membrane that can separate nitrogen from methane by exploiting an important difference between the molecules: their shapes. Nitrogen is a linear molecule, whereas methane is tetrahedral with a trefoil-shaped profile.These membranes could be an energy-efficient and cost-effective alternative to the current natural-gas valorization methods. The use of precise pore editing to separate compounds on the basis of shape could be applied to other complex and challenging processes for which the resources cannot be separated using conventional approaches.
Related papers
- Nature 606 (7915), 706-712
- Nature 4763, 5
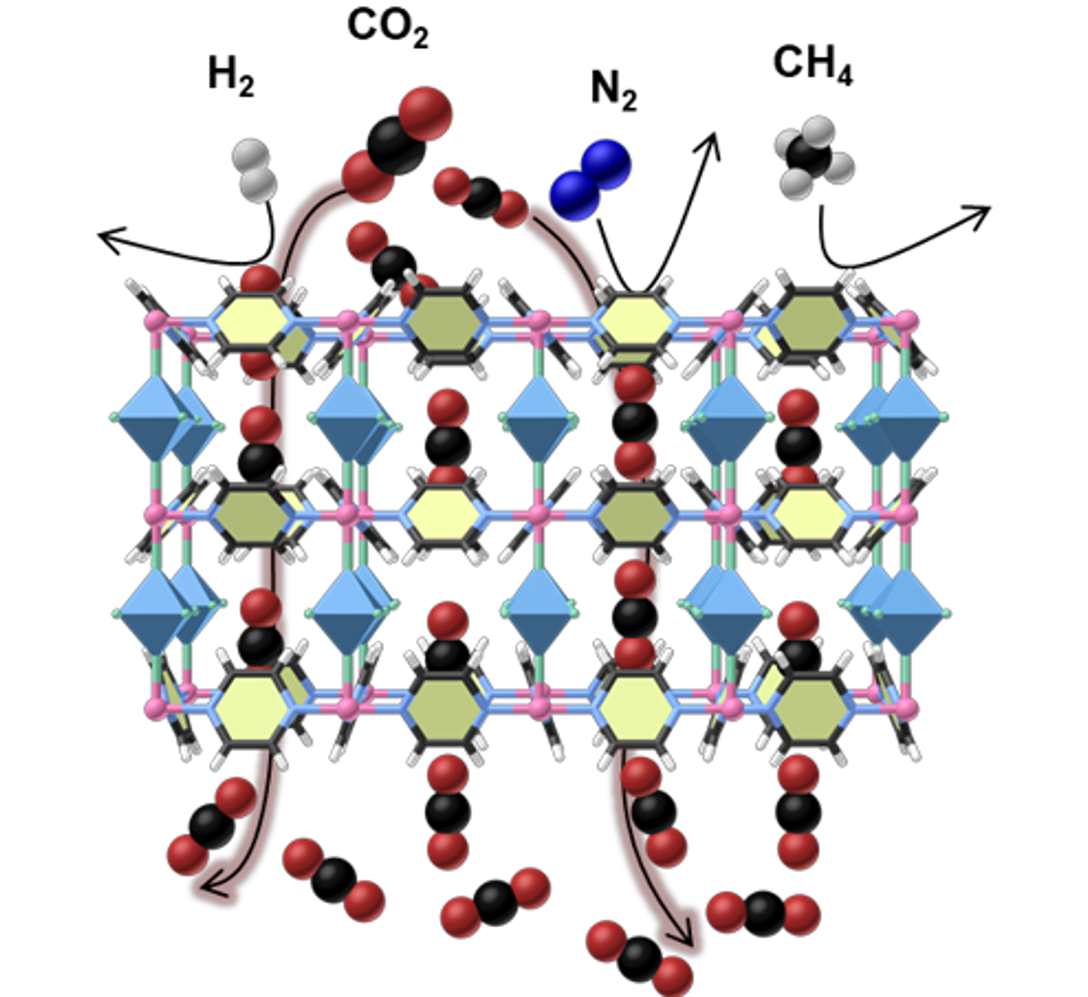
Continuous carbon capture
The steady removal of carbon dioxide (CO2) from diverse gas streams is a critical step to achieve the blueprint of carbon neutrality and for the clean energy production, such as hydrogen (H2) and methane (CH4). However, the associated energy and capital inputs are considerably high, necessitating the development of effective technologies for CO2 separation processes. Membrane-based separation technology offers the desired potential and a constant flux of CO2 would be formed during the membrane permeation, which is driven by the pressure difference. However, challenges associated with the plasticization and trade-offs between the permeability and selectivity for the polymer materials have limited their working range and capacity in the real-world operation conditions. We designed a special CO2-recognition membrane for the continuous and efficient CO2 capture from various mixtures including CO2/H2, CO2/CH4 and CO2/N2. Uniquely, the appropriate CO2 affinity cooperating with the confined aperture enables the membrane to be nearly only permeable to CO2, thus showing unprecedented CO2 separation selectivity over both smaller (H2) and larger (N2, CH4) molecules. The extraordinary performance is maintained after treatment with extreme conditions such as corrosive hydrogen sulfide or humid atmospheres, swing temperatures or pressures, and long-term operations, pinpointing the potential for high-throughput CO2 capture in a continuous mode.
Related papers
- Nature 606 (7915), 706-712
- Chem 9, 1182-1194
- Angew. Chem. Int. Ed. 58 (1), 327-331
Mixed-matrix membranes
A mixed-matrix membrane is a type of membrane that is composed of a polymer matrix filled with dispersed particles or fillers. These particles can be inorganic materials, such as zeolites, carbon nanotubes, or metal-organic frameworks, or organic materials, such as graphene oxide or polymers with specific functionalities. The addition of fillers can improve the selectivity and permeability of the membrane, leading to better separation efficiency. The fillers can create additional transport pathways or adsorption sites, allowing for more effective separation of gases or liquids. The fillers can provide mechanical reinforcement to the polymer matrix, making the membrane more resistant to physical stresses, such as pressure or temperature variations. This enhances the membrane's stability and extends its lifespan. By selecting different fillers, the properties of the mixed-matrix membrane can be customized to meet specific separation requirements. For example, zeolite fillers can enhance the adsorption capacity, while carbon nanotubes can improve conductivity. Mixed-matrix membranes can be used in a wide range of applications, including gas separation, water purification, and drug delivery systems. The versatility of these membranes makes them suitable for various industries and research fields.
Related papers
- Chem. Soc. Rev., 2022, 51, 8300-8350
Fine-tuning of porous materials
Fine-tuning of porous materials refers to the process of modifying and optimizing the properties and performance of these materials by controlling their pore structure, surface chemistry, and other characteristics at the nanoscale. Porous materials, such as metal-organic frameworks (MOFs), zeolites, porous carbons, and covalent organic frameworks (COFs), possess a network of interconnected pores that provide a large surface area and high adsorption capacity. By carefully selecting the synthesis conditions and adjusting the reaction parameters, it is possible to control the size and shape of the pores in porous materials. This allows for the customization of materials with specific pore sizes to accommodate different molecules or ions. The surface chemistry of porous materials can be modified by introducing different functional groups or metal ions onto the pore walls. This modification can enhance the selectivity and affinity of the material towards specific target molecules or ions, making it suitable for various separation, catalysis, or sensing applications. Porous materials can be designed to selectively capture and store specific guest molecules within their pores. The encapsulated molecules can influence the properties and behavior of the materials, leading to unique functionalities such as controlled release, drug delivery, or gas storage.
Related papers
- Nature 606 (7915), 706-712
- Nature Energy 6 (9), 882-891
- Science advances 4 (10), eaau1393